Hello Gnees Army,
Good Evening,
A base, in chemistry, is a substance that delivers hydroxide ions (OH−) when dissolved in water or aqueous solution. Many weak bases [there is also some exception] don’t immediately carry hydroxide ions, but they too produce high levels of OH− when treated with water (i.e. when ammonia is treated with water to produce ammonium and hydroxide ion).
Bases also have some common distinctive physical characteristics like – they are bitter in taste (acids are sour) and gives a slippery sensation when touched [Never try to experiment this!!! It can burn your skin instantly]. Bases are essential and are a vital ingredient in our life too. For example, they are used to make paper, soap and synthetic rayon, bleaching powder, antacid, etc. But in this post, we aren’t talking about bases but about superbase – the strongest super base – Ortho-diethynylbenzene dianion. Here we go…
See Also: Fluoroantimonic Acid – The Strongest Acid in the World Ever!
Now, what is superbase and how much powerful it is?
A superbase is a compound that has a high affinity for protons and 2x or more times stronger than normal bases. Superbases are generally made of combining other different bases. Where the very strong base NaOH has proton affinity of 1038 kJ/mol, the hydroxide ion has of 1635 kJ/mol…Here the strongest superbase (strongest possible organic base) in the world Ortho-diethynylbenzene dianion has a missive proton affinity power of 1845 kJ/mol.
[Proton Affinity: The amount of energy released when a compound bond with hydrogen]
Read Also:
- Why doesn’t empty space pull the Earth’s atmosphere?
- Why there are three lights on the keyboard? What it’s Meaning?
- Kingda Ka – The Tallest Roller Coaster in the world 2020!
- What is group insurance and what are the types of them?
- What’s the difference between group and individual insurance?
- Amazing 27 Soft foods to eat after wisdom Teeth Removal
- Jeffrey Dahmer – AMERICA’s one of the Most Notorious Serial Killer Ever!
It can be called as a super-powerful than powerful!!! Now you can imagine how strong it is!! However, The term superbase is not new and has been used from the 1850s or more. Since superbases are sensitive to a violent reaction, when coming into contact with water or atmospheric carbon dioxide, and, often, oxygen, a special solvent is required to carry chemical reactions. [Inert atmosphere techniques and low temperatures minimize these side reactions.]
One thing to notice here – No suitable solvent exists for these exceptionally strong bases, so they are synthesized in the gas phase conditions that allow direct observation of certain reactions and calculations of their proton affinity. Here the list of the some top strongest superbases ranked by their calculated gas-phase basicity (proton affinity) –
- ortho-Diethynylbenzene dianion
- meta-Diethynylbenzene dianion
- para-Diethynylbenzene dianion
- Lithium monoxide anion
- Methyl anion
Now which will be called base and superbase?
[Difference between Base and Superbase]
Basicity or Acidity of any compound is generally measured in pH scale (pH value ranges from 0 to 14). Where less than 7 means the compound is acid and the greater than 7 is base. So likewise, the pH 0 of any compound will be called strong acid and pH 14 will be a strong base.
But when the measuring of basicity of any compound is greater than 14, there the pH scale is not enough to measure that extremely high base. In general, when any compound level up this measurement is called superbase. Subsequently, this compound now capable of deprotonation (Capability to completely react with water means split hydrogen ions from the water molecules). And for base power measurement is done with their Proton Affinity.
Ortho-diethynylbenzene dianion
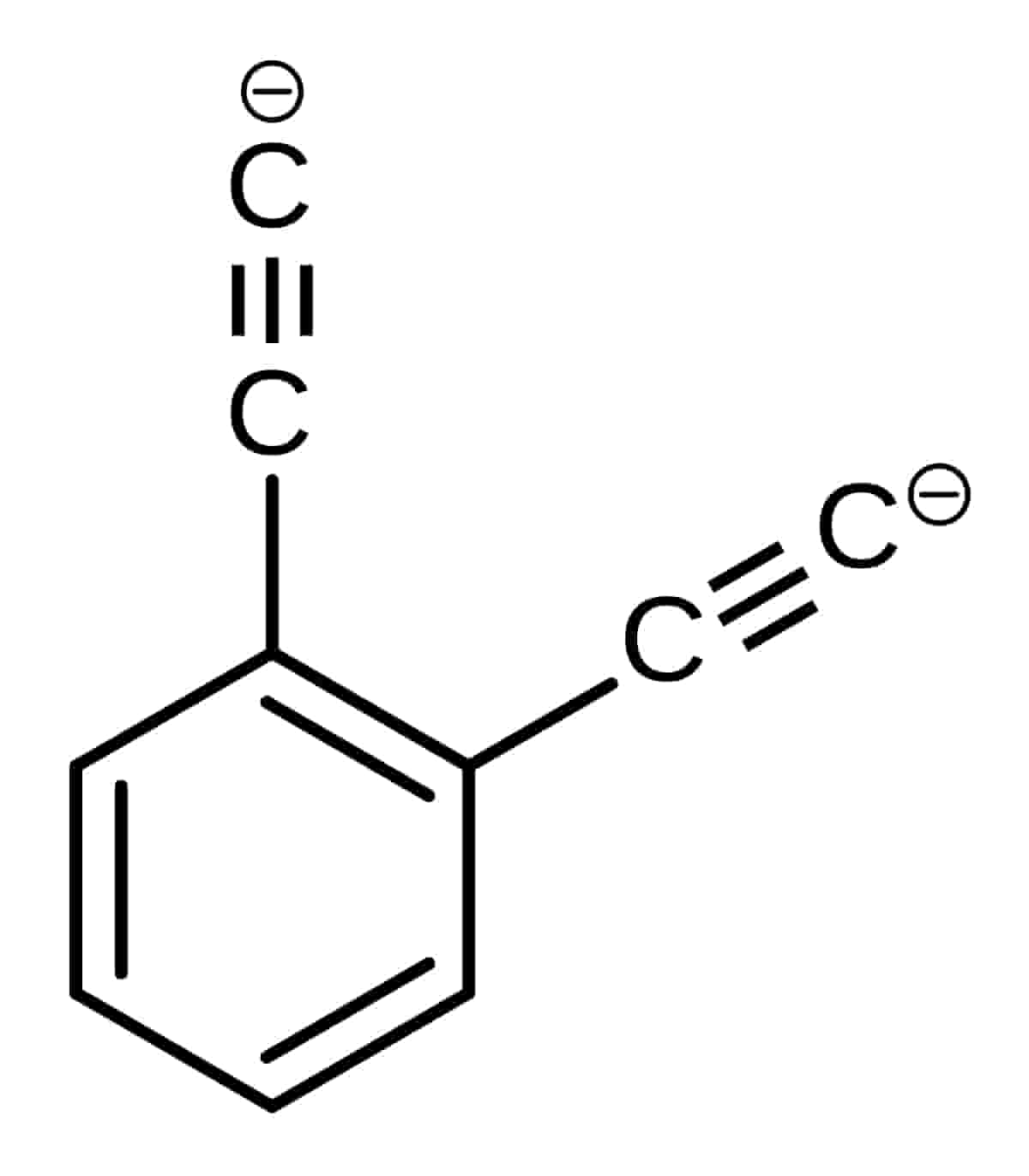
The chemical formula of ortho-diethynylbenzene dianion superbase is [C6H4(C3O2)2]2−. It was created in mass spectroscopy experiments by researchers in Australia. Each of the ethynyl groups in this base has a negative charge. Till now Ortho-diethynylbenzene dianion compound has no known commercial use yet. But in the future, it could have various applications in industry, including the battery industry, as a component in alkaline batteries.
However, It has two isomers: Meta-diethynylbenzene dianion and Para-diethynylbenzene dianion. All of these isomers including Ortho-diethynylbenzene dianion exist in the gas phase, contrary to the normal bases (such as the hydroxide anion which exists in the solution/aqueous state).
The methyl anion H3C– was the strongest known base for 30 years, until Tian and his colleagues made the lithium monoxide anion (LiO−) in 2008, which has held the record since. Up until 2008, the Lithium monoxide anion (LiO−) was the strongest Superbase. But like most of the bases are found in the solution state, it’s not existed in the solution state, rather in an aprotic state (An aprotic solvent is a solvent that has no O-H or N-H bonds) means non-aqueous solvent.
Ohh but unfortunately it will be not the king anymore. Now, scientists in Australia have knocked LiO– down to the second place, making a gas-phase dianion with the highest basicity ever found in the world. This compound is so much powerful that it can even deprotonate one of the stable compound benzene.
Its uses and importance
Although for now, this type of molecule is often too unstable to exist due to repulsion between its adjacent negative charges, in future this problem may be solved by chemists.
In conclusion mass production of it in future will impact commercially on important areas of chemistry such as petroleum reforming, polymerization catalysis, battery technology and fuel cell technology.
How it is named Ortho-diethynylbenzene dianion?
Ortho-diethynylbenzene dianion – The IUPAC chemical name is derived from the functional groups which make up the compound.
Here, the name Benzene is used as the compound has a benzene ring.
diethynyl for the compound has two ethynyl functional groups as elements.
dianion is mentioned as the compound has a charge of – 2 (di- is two and anion refer to compounds having a negative charge)
And Ortho is used for –
There are two functional groups (di means two) in the compound which is the ‘-ethynyl ‘ group. Counting the number of Carbon atoms in a clockwise manner, one of the ‘-ethynyl ‘ groups forms a bond with the first Carbon atom. Where the other ‘-ethynyl ‘ group forms a bond with the second Carbon atom in the benzene ring.
Thus this forms aromatic compounds which have two functional groups bonded to the Carbon atom and the Carbon atom to which one of the functional group bonds with is known as the First Carbon atom. It is used as a starting point to number the other Carbon atoms.
Notice here to know that when the other functional group bonds with a second / third / fourth Carbon atom, the IUPAC name of the compound takes the following prefix: ortho- / meta- / para- respectively. In this compound, the second functional group (‘-ethynyl ‘ group) bonds with the second Carbon atom. That’s why the compound takes the name Ortho- as per IUPAC naming. Hence, the IUPAC name of the compound is Ortho-diethynylbenzene dianion.
Hope you enjoyed this post. Thanks for reading! See you again 😉
Read Also:
- Fluroantimonic Acid – Meet the strongest acid in the world!
- What is pneumonoultramicroscopicsilicovolcanoconiosis?
- Painite – The Rarest Gem on Earth! | Price $50k/0.2 gram
- Paedophryne Amauensis – Meet the smallest frog in the world!
- 1960 Valdivia Earthquake | Biggest Earthquake in the world!
- What is the fastest bird in the world? The Fastest Ever!!
- What is the premium in insurance? Different Premiums?
- How many types of Insurance are there?
- How many types of play button on YouTube?


